Material
|
Catheter Length
|
Size
|
 |
CE is an abbreviation for Conformite Europeenne (European Conformity) and the mark indicates that all the requirements for the specific product meet the European standards and that it has been tested and allowed to enter the European market. Learn more: http://ec.europa.eu/growth/single-market/ce-marking/ |
 |
ISO (International Organization for Standardization) 13485:2003 standard represents the quality management system for design and manufacturing of medical devices; it was published in 2003. This international certification requires the manufacturer the quality system of the facilities. Learn more: http://www.iso.org/iso/catalogue_detail?csnumber=36786 |
 |
FDA (the Food and Drug Administration) is an agency of the United States responsible to protect and regulate public health products, such as medical products, tobacco, food, veterinary medicines. Learn more: http://www.fda.gov/ |
About Our Nelaton Catheter
Why StayDry?
Consumer Tips

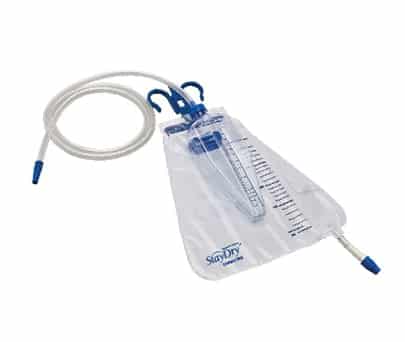
AdvaCare’s Nelaton Catheters is designed differently from other similar catheters, currently available. The unique feature is the connector which incorporates a cap that fits in the connector funnel. The angular Tiemann tip (ball tip) enables the prostate gland to be circumnavigated in an especially careful manner. A mark on the connector shows the alignment of the Tiemann tip whilst it is being inserted. The cap can be easily detached with a slight tug if the users want to remove it. This provides the user means of interrupting urine flow any time during catheterization. Frozen surface tubing for super smooth intubation.


Why Our Nelathon Catheter?
AdvaCare’s StayDry™ Nelaton catheter incorporates a very smooth and lubricant coating that ensures maximum comfort during insertion and removal. AdvaCare’s Nelaton catheter is manufactured with very high standard manufacturing process looking after the safety and comfort of the users.
Cautions
- 1 – This product should be used solely for intermittent catheterization, upon the recommendation of a qualified healthcare practitioner;
- 2 – Do not reuse as this product is meant for single use only;
- Avoid using the product if there are signs that the packaging has been tampered with;
- 3 – Use product immediately after opening. After usage properly dispose of the medical equipment by placing the device in the opened packaging and disposing of it responsibly.